Polio is an infectious disease, contracted predominantly by children, that can lead to the permanent paralysis of various body parts and can ultimately cause death by immobilizing the patient’s breathing muscles.
No cure exists for the symptoms, but in the 1950s effective vaccines were developed and have been used around the world since then. This allowed some richer countries to eliminate the disease entirely in the 1960s and 70s. But large outbreaks continued around the world and in the 1980s the estimated global number of paralytic cases was over 350,000 per year and the disease was still prevalent in 125 countries. As a response the “Global Polio Eradication Initiative” (GPEI) was founded in 1988 to fight the virus’s spread and disease burden with a global vaccination campaign. Since then the world has made rapid progress against the disease and until 2016 the number of paralytic cases was reduced by 99.99% with 42 cases in that year worldwide. The latest data on the number of polio cases is always up-to-date here.
As of 2017 the virus remains in circulation in only three countries in the world – Afghanistan, Pakistan and Nigeria – and it is hoped that the disease will soon be eradicated globally.
All our charts on Polio
Polio, short for poliomyelitis, is an infectious disease that is caused and transmitted by a virus called the poliovirus. One of the severe symptoms of polio is paralysis and the disease is therefore also known as “infantile paralysis”. The name poliomyelitis is derived from Greek and translates to gray (polios) marrow (myelon), which refers to the tissue in the center of the spinal cord, which when affected causes paralysis. Paralyzed limbs such as arms or legs waste away over time which is the cause of deformed child legs most commonly being associated with the disease polio.
Permanent paralysis fortunately occurs in only 0.5% of infections. The majority of infections (72%) do not lead to any symptoms. About a quarter of cases (24%) result in “abortive” poliomyelitis which leads to nonspecific symptoms for a few days, such as a fever or a cold, and 1-5% of cases lead to “non-paralytic aseptic meningitis”, in which the patient suffers from stiff limbs for up to 10 days.1
The poliovirus is found only among humans and is transmitted via the so-called fecal-oral route. In other words, polio is mostly transmitted by drinking water that has been contaminated by the feces of a person carrying the poliovirus. The virus therefore spreads especially well in conditions of poor sanitation, for example when people defecate in the open or do not filter their water before drinking it. The fact that the virus can only survive in humans (and no other animals) makes it possible to completely eradicate the disease from the world – if it was a virus with an animal host such as influenza (birds) or tuberculosis (cows) that occasionally mutates to attack humans, polio could only ever be controlled but not eradicated.
Its relatively long incubation period of up to 10 days and three fourths of infections not showing any symptoms makes polio extremely difficult to monitor and the virus can spread for several months without being detected. Monitoring has to focus on identifying patients that suffer from symptoms or rely on stool samples. Because single identified cases might indicate larger outbreaks, the WHO recommends to treat such cases as a public health emergency when only one child is identified with a wild, endemic polio infection in a country that was previously declared polio free.2
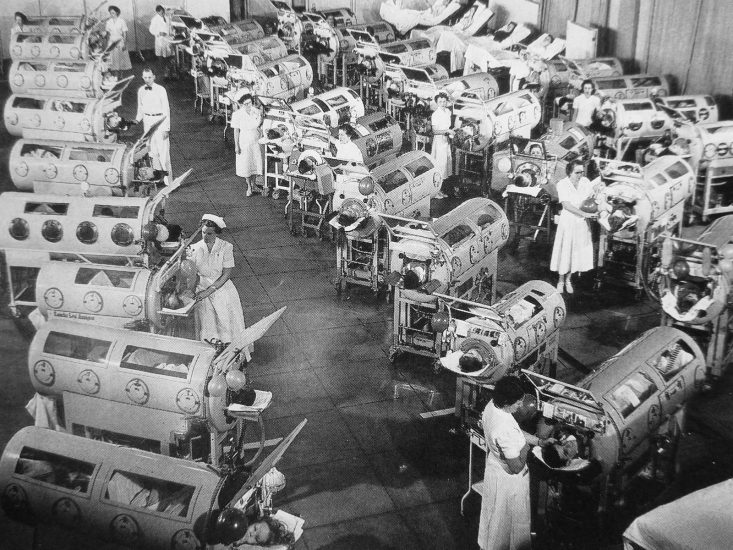
Polio can lead to the death of infected patients when the paralysis is immobilizing their breathing muscles.3To prevent the death by suffocation, Harvard professors Philip Drinker and Louis Agassiz Shaw invented the so called “iron lung”4 (shown in the picture) in 1928. Infected patients would be placed in an air-tight tube – with their heads outside – and the machine decreased the pressure inside the box, to induce inhalation, before returning to the normal outside pressure conditions, to induce exhalation. Most patients would spend one to two weeks in an iron lung before their paralytic symptoms faded and independent breathing was achieved once again. In the case of permanent paralysis, on the other hand, patients would be bound to live inside the iron lung for years.
As it is often the case with innovations, the iron lung became only widespread when the price declined substantially. John Emerson managed to construct an iron lung at half the cost in 1931. In the meantime, Philip Drinker had tried to protect his invention with a patent and therefore filed a lawsuit against Emerson for using the original iron lung set-up. Emerson did not succumb, claiming that life-saving devices should be made available to the public at low cost rather than be used for private financial gains, and in the end, Drinker’s lawsuit failed.5
The falling price of the Iron Lung enabled mass distribution and widespread use from the end of the 1930s onwards. The cost of one iron lung in the 1930s amounted to US-$1,500, which is the equivalent of approximately US-$26,000 in 2016 when accounting for inflation.6
The design quickly spread to Western Europe and was widely used for polio patients there, too.
The data visualization further below, which shows the prevalence of polio in the US over the last century, shows two large outbreaks in 1916 and 1952. It can be seen that in 1916, before the widespread use of iron lungs, the death rate increased sharply when the case rate increased. But after the widespread use of iron lungs from 1939 onwards and during the largest polio outbreak in the US in the 1950s the death rate did not show the same strongly correlated increase anymore. This is because the breathing assistance of the iron lungs prevented the deaths of the many polio patients suffering from paralyzed breathing muscles – the iron lungs were a large step forward in mankind’s struggle against polio.
A hospital room in the US with patients in iron lungs in 19527
Historical Perspective
The history of polio can be divided into three major phases:8
- The endemic phase from antiquity to the nineteenth century in which the disease occurred relatively rarely and did not result in many paralytic cases.
- The epidemic phase until the mid-20th century, during which the world saw large-scale outbreaks and increased geographic spread.
- And the vaccine phase that followed the introduction of vaccines in 1955. In this phase polio prevalence has been reduced first in richer countries and over the last decades in poorer countries around the world.
The hope is that the world will see a fourth and final phase, in which polio is entirely eradicated from the planet.
Infectious diseases are generally believed to arise from the interplay of various developments such as humans’ settlement in urban structures, crowding that causes poor hygiene, food shortages enhancing populations’ morbidity, and the domestication of animals. The exact origins of the disease are not known, but based on the characteristics of the disease, epidemiologists are able to hypothesize how the disease has evolved and spread.9
Because the disease requires a human host and does not survive outside the human body for longer than one to two weeks, the disease could only have developed when humans started to settle in larger agglomerations. Various skeletons have been found with deformations similar to polio10 but the most widely-referenced indication of polio has been an Egyptian stele (pictured) depicting Doorkeeper Roma with one leg skinnier and deformed, both typical symptoms of paralytic polio.
An Egyptian Stele dating back to 1403-1365 BC of a man with polio11

Since then, evidence of polio occurrences are scarce. But in different times records to polio-like diseases appear. The poet Sir Walter Scott was infected with polio at the age of eighteen months in 1773 and the disease paralyzed his right leg for life. In his Treatise on the Diseases of Children in 1789 the London pediatrician Michael Underwood writes about a children’s disease, possibly polio, that is a “debility of the lower extremities”.12
Known outbreaks in the 19th century recorded no more than 30 cases as can be seen in the table, taken from Smallman-Raynor & Cliff (2006).13
Polio-like outbreaks in the 19th century14
| Year | Location | Cases (deaths) |
|---|---|---|
| 1808 | Göteborg, Sweden | 4 (-) |
| 1835 | Worksop, UK | 4 (-) |
| 1841 | Louisiana, USA | 10 (-) |
| 1868 | Modums, Norway | 14 (4) |
Up to the 19th century, populations experienced only relatively small outbreaks. This changed around the beginning of the 20th century. Major epidemics occurred in Norway and Sweden around 1905 and later also in the United States.15
Why did we see such large outbreaks of polio only in the 20th century? Or, in other words, why did the transition from the endemic to the epidemic phase take place?
The answer, again, lies with hygiene standards. As polio is transmitted via the fecal-oral route, the lack of flush toilets and the lack of safe drinking water meant that children in the past were usually exposed to the poliovirus before their first birthday already. At such a young age, children still benefit from a passive immunity that is passed on from their mothers in the form of antibodies. These are proteins that identify the poliovirus as something foreign and therefore signal to the body that they should be eliminated (done by either the antibodies themselves or different cells called macrophages). Thereby, virtually all children would contract the poliovirus at a very young age. While protected from developing the disease thanks to the maternal antibodies, their bodies would produce their own memory cells in response to the virus and that ensured long-term immunity against polio. The latter is important as the mother’s cells have a half-life of only 30 days (starting from the last day of breastfeeding).16
Once the maternal antibodies decrease in number, children lose their passive immunity.
As hygiene standards improved, the age at which children were first exposed to the poliovirus increased and this meant that the maternal antibodies were no longer present to protect children from polio.
That improved sanitation was a driving factor in polio outbreaks is also illustrated by the fact that the age at which polio was contracted increased over time. During five US epidemics in the time period 1907-1912, most reported cases occurred in one- to five-year-olds, whereas during the 1950s the average age of contraction was 6 years with “a substantial proportion of cases occurring among teenagers and young adults”.17
Being exposed to the poliovirus after losing the protection from maternal antibodies meant that they were more likely to get polio, resulting in an increase in case and death numbers around the turn of the 19th and 20th century.
The chart here shows the annual absolute number of reported deaths and cases in the United States over the last century; the corresponding perspective on the rate of deaths and cases is shown in this visualization. Big outbreaks happened frequently. In 1916 for example, the poliovirus infected more than 27,000 Americans and killed more than 7,000 people. At that time, the cause and spread of the disease was not yet known, so panicked New Yorkers shut down schools, public cinemas, and swimming pools. For a while it was believed that cats or mosquitoes were spreading the virus which lead to the killing of more than 72,000 cats and the extensive spraying of the insecticide DDT, in a futile attempt to interrupt the transmission of the virus.18
Each of these large outbreaks came to an end because, like most viral diseases, the spread of the poliovirus in moderate climates like in the USA is seasonal and is mostly transmitted during the summer months. By October 1916, enough New Yorkers had been infected and developed immunity in response so that in combination with the natural seasonal decline of the virus’s spread the case numbers had already dramatically dropped and would not surge again. This can be seen in the graph as the US recorded less than 5,000 cases in the following year. Containing the second major outbreak in the USA in the 1950s, on the other hand, was largely aided by the successful development of polio vaccines that would hinder the transmission of the virus.
The vaccine against polio
What changed the history of polio forever was the development of a vaccine against the disease. US President Franklin D. Roosevelt himself had been diagnosed with polio at the age of 39 and subsequently bound to a wheelchair for the rest of his life. While this might have been a misdiagnosis in Roosevelt’s case,19 his presidential influence was crucial in the set-up of the National Foundation for Infantile Paralysis. The non-profit organization soon became known as “The March of Dimes Foundation”, referring to polio victims’ inability to walk, and successfully collected a substantial amount of donations for vaccine research and its ‘Iron Lung’ distribution program.
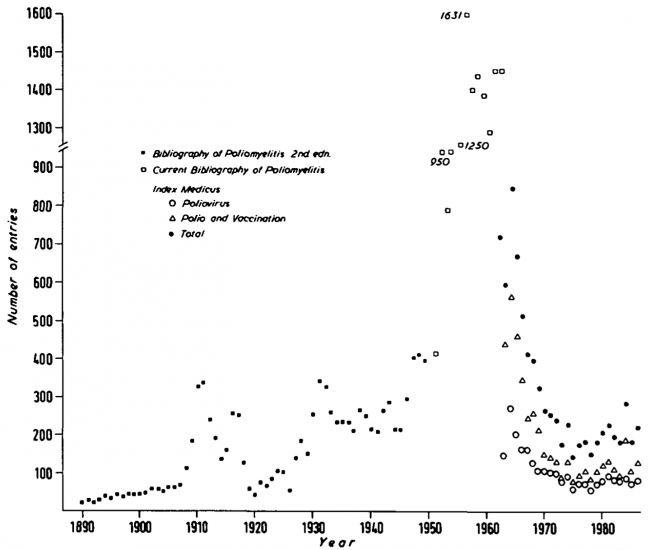
Years of research went into the effort to develop an effective vaccine. The chart shows the large increase in scientific publications on polio in the 1950s.
The medical doctor and virologist Jonas Salk put forward a promising vaccine and in the spring of 1953, the foundation rolled out a large-scale trial of the Salk vaccine for which 1.83 million children in 44 US states received either a placebo or the vaccine shot.20 Salk’s supervisor Thomas Francis insisted to introduce a control group into the trial design and thereby paved the way for Randomized Control Trials, which have become a very influential method in medical and social science research in the following decades. The foundation was supported mainly by donations from the American people, who were collecting dimes, quarters, and dollars for decades in the hope of research finally uncovering a way to protect oneself against polio. Oshinsky (2005) even reports that the foundation received donations from two-thirds of the US population and a poll claims that more Americans knew about the field trials than about the president’s full name (Dwight David Eisenhower).21
On April 12, 1955, the tenth anniversary of Roosevelt’s death, Francis announced that Salk’s vaccine was effective and potent in preventing polio. Within just two hours, the US Public Health Service issued a production license and the foundation prepared for a national immunization program. The conference had been live-broadcasted to physicians all over the country who had gathered in movie theaters to watch the announcement, millions of Americans received the news over the radio, spontaneously putting down their work in celebration of the news.22
At 10:30PM of the same day, Thomas Francis and Jonas Salk gave a televised live broadcast interview in which Salk, when asked who owned the patents to the vaccine, famously answered “Well, the people I would say. There is no patent. Could you patent the sun?”23
His answer was in the spirit of the foundation having funded the vaccine research with American’s private donations and his conviction that life-saving technology should be for the benefit of society as a whole rather than for private financial gains.
Shortly afterwards Dr. Albert Sabin introduced a live polio vaccine that could be administered orally (rather than Salk’s by injection), the oral polio vaccine (OPV).24
While Salk’s vaccine only protected the central nervous system, Sabin’s vaccine also protected the digestive tract and thereby prevented the spread of the wild polio virus more effectively. The easier administration also made vaccination efforts less expensive as it did not require trained health workers. For these reasons, OPV has been used around the world and it is the vaccination that is responsible for the dramatic reduction in polio infections globally that we document below.
The graph of the number of reported polio cases and deaths in the United States shown above shows the impact the national immunization scheme had: Within 7 years, the number of reported polio cases went below 1,000 and by 1979 the US was declared polio-free. It is easier to see this decline if you switch the y-axis in that chart from linear to logarithmic.
Numbers of publications about polio by year, 1890 to 1986 – Cambridge World History of Human Diseases25
Unfortunately it is possible that the altered live poliovirus that is used in the oral polio vaccine (OPV) mutates and thereby regains its “neurovirulence”. Thankfully it happens extremely rarely (more below), but it means that recipients of the OPV can develop the paralytic symptoms from the vaccine.
There exist two types of vaccine-induced polio, VAPP and cVDPV:
- If the mutation is spontaneous, one refers to Vaccine-Associated Paralytic Polio (VAPP) which is not infectious. It is estimated that it occurs once per 2.7 million doses of OPV.26
- If the mutation happens over the course of at least one year by transmission among an under-immunized community, the virus can transform to resemble the wild type of poliovirus. This is then known as a circulating Vaccine-Derived Poliovirus (cVDPV) and this virus can be transmitted via the fecal-oral route to other humans. Since 2000, 10 billion doses of OPV have been distributed worldwide and only 24 cVDPV outbreaks have occurred, counting less than 760 cases.27
As the attenuated vaccine virus requires conditions of low vaccine coverage to develop into a VDPV, it is not the vaccine itself, but low immunization rates that pose the problem.
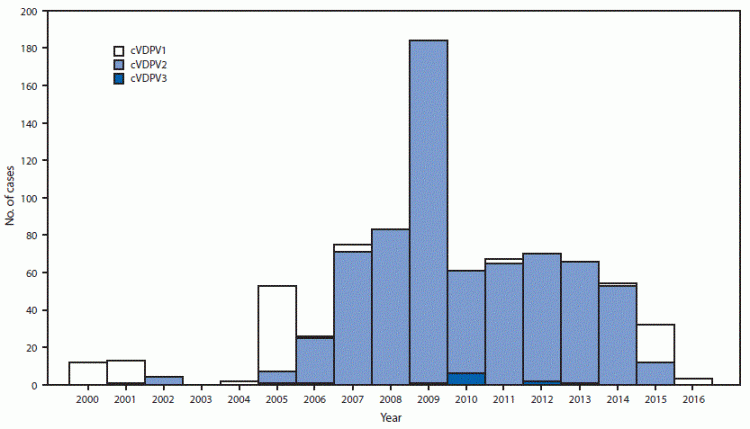
The chart shows the number of circulating Vaccine-Derived Poliovirus (cVDPV) outbreaks between 2000 and May 2016. More than 90% of the vaccine-derived viruses were of serotype 2, a variation of the virus whose wild-type equivalent was already globally eradicated in 1999. Therefore, the Global Polio Eradication Initiative switched from an OPV that protects against all three serotypes to one that only protects against serotypes 1 and 3 in April 2016 and this has led to a dramatic decline in vaccine-derived polio viruses.
Further below, in section II.4 The benefits of eradicating polio, we show that since 1988 16 million cases of paralytic polio have been averted thanks to the Global Polio Eradication Initiative’s (GPEI) vaccination campaigns.28
Moreover, the initiative has declared it its goal of switching to the inactivated poliovirus vaccine (IPV), an updated version of the Salk vaccine, which is administered by injection and does not bear the risk of vaccine-derived polio strands.
The global number of circulating Vaccine-Derived Poliovirus (cVDPV), January 2000 – May 201629
Dr. Sabin’s Oral Poliovirus Vaccine (OPV) was tested on more than 100 million people in the Soviet Union before obtaining its license in 1961. Because its production costs were lower and the oral administration easier, OPV was and still is the predominant vaccination serum in many countries. It was only during the 1970s and 1980s with several polio surveys throughout the developing world that became known as “lameness surveys” that it became apparent that polio was as much a problem in developing countries as it was in Western Europe and the United States. Bernier (1984)30 cites 46 of these “lameness surveys” in 24 countries, with a prevalence of paralytic polio ranging from less than one to 19 cases per 1,000 children. Modlin (2010)31 claims that these countries suffered from a higher prevalence of polio than the USA during its peak polio outbreaks.32
Mass campaigns in Brazil, Cuba and Mexico proved the vaccine’s effectiveness in different geographical areas but only with the foundation of the Global Polio Eradication Initiative at the World Health Assembly in 1988 did the polio vaccine find its place in many national routine immunization programs.
This visualization here shows how the polio vaccine coverage of one-year-olds has substantially increased around the world since 1980. By switching to the chart view you can see the change over time for each country and the world as a whole. Globally you can see that in 1980 only 22% of one-year-olds were vaccinated against polio, and this increased to a coverage of 86% of the world’s one year-olds in 2015.
Global decline of polio
In 1988, the World Health Assembly – the governing body of the World Health Organization (WHO) – launched the Global Polio Eradication Initiative (GPEI) which was tasked with eradicating the disease globally by the year 2000.33 The eradication of the disease in just 12 years was an ambitious plan, polio was still endemic in 125 countries of the world in 1988.
The GPEI was set up as a public-private-partnership and today brings together several organizations, among which are the WHO, UNICEF, the US Center for Disease Control and Prevention (CDC), Rotary International and the Bill and Melinda Gates Foundation.34
As can be seen in the graphic depicting the decade of the last recorded paralytic polio cases below, several countries had either eradicated or were very close to eradicating polio in 1988. They had achieved that by way of routine immunization programs which entails the basic schedule of each child receiving three Oral Polio Vaccines (OPV) before its first birthday. Since its inauguration in 1988, the GPEI has offered support for these routine immunization programs to governments, but in addition to these the GPEI also ran
- National Immunization Days (NIDs), on which children receive two doses of OPV 4-8 weeks apart regardless of their immunization history,
- outbreak response immunization programs, for which all children below the age of five years in the vicinity of a detected case of paralytic polio receive one OPV dose, and
- mopping-up immunization programs, during which under-five-year-olds living in outbreak-prone areas are visited in their homes and receive two OPV doses one month apart.
Even though the GPEI has not yet reached the goal of eradicating polio, it has been successful in reducing the prevalence of polio around the world: reported polio cases have been reduced by 99.99% and two of its three serotypes have already been eradicated. In 2017 the polio virus is endemic in three countries only: Afghanistan, Nigeria, and Pakistan – colored in dark red in the map. Up-to-date information on polio is available on the website of the GPEI here.
Because of the increasing burden of vaccine-derived paralytic polioviruses (cVDPV), the GPEI has switched from the oral polio vaccine (OPV) against all three strands of poliovirus (see our explanation of cVDPVs above) to a “bivalent” OPV against only strands 1 and 3. Furthermore, in 2012 the initiative announced its goal of entirely phasing out orally-administrated vaccines (OPVs) and use only injection-administrated vaccines (IPVs) instead as part of the Polio Eradication & Endgame Strategic Plan 2013-2018. This way, they hope to not only eliminate the wild poliovirus type but also the vaccine-derived poliovirus types to eradicate polio from this planet in its entirety.
The map displays the year of the last recorded case of polio for each country and each decade is color-coded. You can see that the Americas were the first world region to be certified polio-free in 1994.
The WHO certifies not individual countries but only entire world regions as polio-free. The considered world regions are the six WHO world regions: Africa, Americas, Eastern Mediterranean, Europe, South-East Asia, and Western Pacific.
To be certified polio-free, a WHO region needs to (i) record no wild indigenous polio case for at least three years, (ii) have a reliable surveillance system in place, and (iii) prove its capacity to detect and respond to imported polio cases.35
The interactive map shows for each country when the last case of endemic paralytic polio was recorded. The map also shows when the four polio-free WHO regions achieved this status, three years after the last country in that WHO region recorded the last endemic polio case.
The interactive visualization highlights the global decline in the number of paralytic polio cases from 1980 onwards. In the early 1980s more than 350,000 people suffered from paralytic polio cases every year, in 2016 this number was only 46 paralytic polio cases. In the 1980s the world saw 20-times as many paralytic polio cases every day than today in an entire year.
The cases are shown for each of the six WHO world regions and you can change the view from absolute to relative numbers of polio cases by clicking on “Relative” at the bottom left of the graphic. In the 1980s between 50% and 75% of all estimated cases occurred in the South-East Asia region, this region has not recorded a single case after 2011 and was certified to be polio free in 2014.
This data of the estimated total number of paralytic polio cases is based on the number of reported paralytic polio cases. Our estimations of the total number follow the methodology by Tebbens et al. (2011)36 which estimates how widespread underreporting – especially in earlier periods – of polio is and then corrects the number of paralytic polio cases to arrive at the estimated true number of cases. For many countries, Tebbens et al. apply a correction factor of 7, which means that they multiply the number of reported paralytic polio cases with seven to arrive at the true number of paralytic polio cases per country. We discuss how we applied the methodology of Tebbens et al. (2011) and extended their study to 2016 and for all countries in the world in a separate document here.
The World Health Organization relies on a similar methodology when estimating the total number of cases. This visualization by the WHO compares the number of reported cases with the number of estimated cases and arrives at very similar estimates to ours with the estimated global number of paralytic polio cases being above 400,000 in the 1980s.
The visualization documents the number of reported polio cases for individual countries from 1980 onwards. You can add and delete countries from the chart yourself by clicking on “ Add country ”.
The data here shows not only endemic polio cases but also cases that were imported from other countries as well as vaccine-derived polio. This is why the chart shows cases even for times at which a particular country had already eradicated polio. This is why the United States, which eradicated polio in 1978, for instance, still record some polio cases in the 1980s.
Because global data are only available from 1980 onwards, the time series of countries which only recently achieved their polio-free status such as India or the three countries in which polio is still endemic in 2017 – Afghanistan, Pakistan and Nigeria – are especially interesting to look at here.
The interactive map presents the number of polio cases per 1 million inhabitants of each country, to account for differences in the population size and make comparisons between countries more meaningful.
You can press play in the bottom left corner to show the change over time. It can be clearly seen how the WHO regions achieved their polio-free status, the Americas for example were certified in 1994.
By clicking on any country you can see the change over time of the polio rate in that country.
The costs and benefits of eradicating polio
The Global Polio Eradication Initiative estimates that their eradication campaign that added National Immunization Days (NIDs), outbreak response immunization and mopping-up immunization to countries’ routine immunization programs as explained above averted 16 million cases of paralytic polio since its inauguration in 1988. In addition, the GPEI also estimates that abandoning its eradication efforts and administrating polio vaccinations only through countries’ routine immunization programs now would increase the number of paralytic cases within ten years back to approximately 200,000 cases per year.37
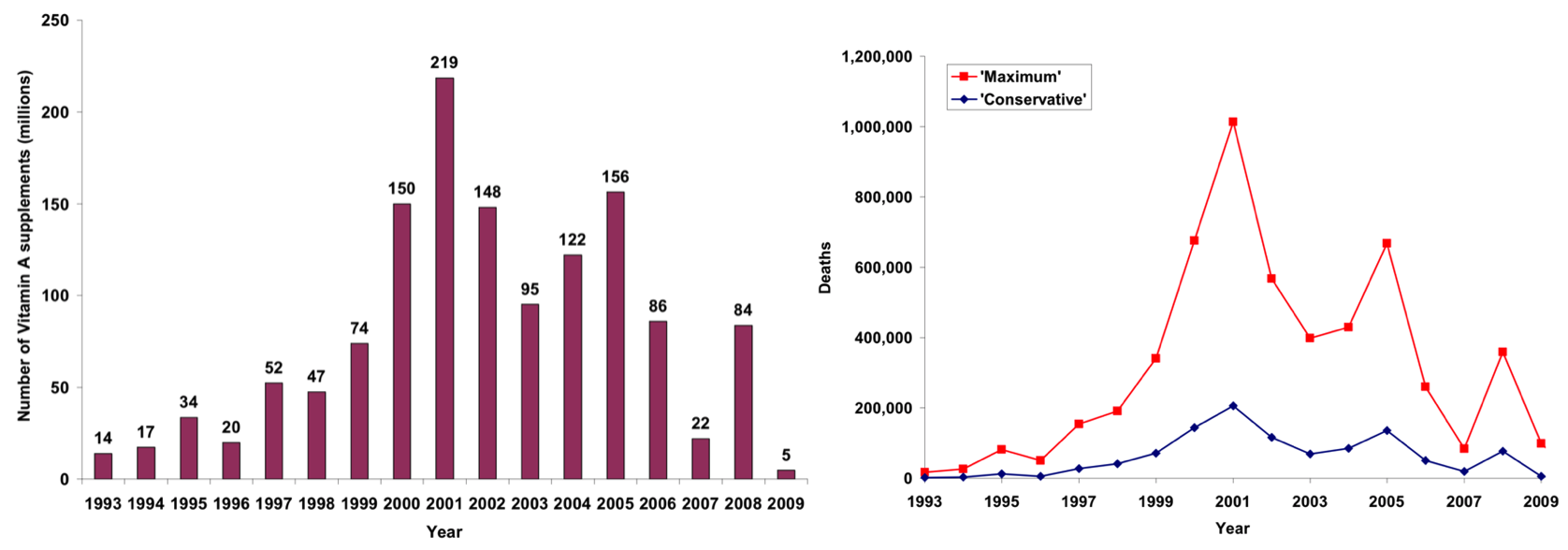
In addition to preventing paralytic polio, many children around the world have received other health benefits that were made available to them as part of the polio immunization campaigns. These are referred to as supplementary immunization activities (SIAs) where other vaccinations and nutritional supplements are distributed. Tebbens et al. (2010)38 estimated these additional side benefits in terms of the reduced childhood mortality, the graphs of which are reproduced here. It can be seen that 219 million supplements were distributed as part of the polio SIAs in 2001 alone and that more than 200,000 lives were saved that year according to their most conservative estimations.
Left: Number of Vitamin A supplements distributed along with polio immunization in Tebben et al.’s (2010) model;
Right: Estimated annual number of prevented deaths due to Vitamin A administration39
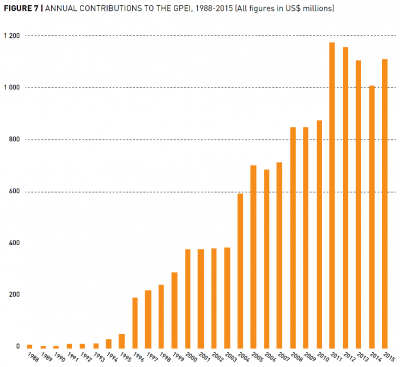
The GPEI received US-$15.2 billion in donations from 1985 to 2019.40 The large majority was only received from 2004 onwards, as the graph from their Financial Resources Requirement Report in 2016 shows. This funding contributed to additional polio eradication efforts in the form of National Immunization Days (NIDs), outbreak response immunization and mopping-up immunization.
Current expenditure on polio eradication and the Endgame Strategic Plan
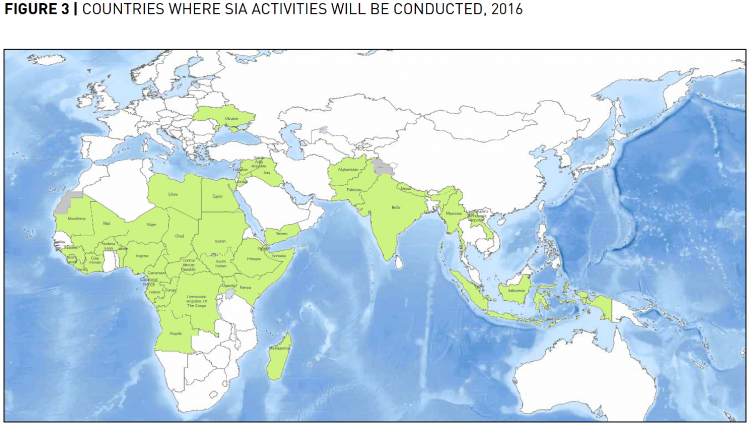
In 2013, the GPEI launched an ambitious five year plan to fully eradicate polio called Polio Eradication & Endgame Strategic Plan 2013-2018 which would cost US-$5.5 billion (in 2015 it was increased by a year and extended to US-$7 billion), also see the section on the benefits of eradication rather than reduction for more information. Even though polio was endemic in only Nigeria, Pakistan and Afghanistan in 2013, it proved especially difficult to monitor the virus and to reach every child for immunization in these contexts which makes the endgame strategy so expensive in comparison to the GPEI’s previous budget. That’s why, in 2016, US-$536 million of the total GPEI budget of US-$925 million were spent in these three countries. However, the GPEI is still actively supplementing immunization projects in 39 other countries to prevent the poliovirus’s transmission across borders and the disease’s re-establishment.42
The map indicates which these countries are that still receive support from the GPEI; only six of these fund their immunization efforts partially themselves.
From 2016 to 2019, the GPEI intends to spend 80% of its budget (US-$3,062 million) on detecting and interrupting polio which includes their support of OPV campaigns and Supplementary Immunization Activity (SIA) schedules. The initiative’s remaining objectives are to (i) replace the oral with the injection vaccine, funded with US-$431 million, to (ii) contain poliovirus stocks, funded with US-$60 million and to (iii) plan for the transition to a post-polio world, funded with US-$26 million.
To put these numbers into perspective, global malaria financing amounted to US-$2.9 billion in 2015 which was more than twice as large as the GPEI’s budget of US-$1.39 billion one year later in 2016. Or, to give a second comparison, in 2016 the US government spent US-$1,116 billion on major health care projects, a budget that is more than 800 times larger than the GPEI’s global spending on polio in the same year. Even in comparison to the total $7 billion budget for the entire Polio Eradication & Endgame Strategic Plan 2013-2018 the US Department’s health expenditures are 142 times that.44
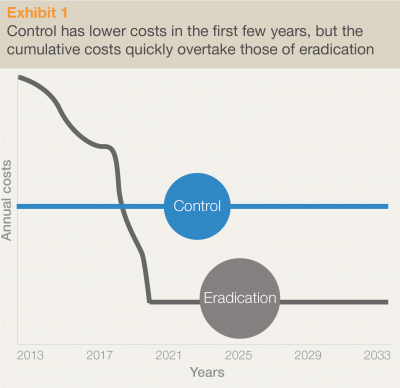
In 2013, the GPEI implemented “The Polio Eradication and Endgame Strategic Plan 2013-2018”, costing an additional $5.5 billion in addition to the already $9 billion spent by the organization since its implementation in 1988. The initiative hopes to especially finance the last stretch of vaccination campaigns in the three countries where polio is still endemic today (Afghanistan, Pakistan and Nigeria), keep the remaining countries, especially in the vicinity of the former, polio-free and closely monitor the occurrence for at least three years after the last reported case. Even though the additional financial needs seem very high, the GPEI argues that eradication is the most cost-effective strategy, by illustrating in their graphic reproduced here that the long-term costs of controlling rather than eradicating will be substantially higher.
The benefits of eradicating polio extend beyond the health domain of having less people suffering paralytic polio. In the economic domain, less polio patients translates into lower healthcare costs. Furthermore, once the virus has been eradicated, the world can stop the production and administration of the polio vaccine as well as the extensive surveillance of paralytic diseases suspected to be polio. The discontinuation of these costly activities will therefore result in extensive economic gains from eradication, as well. It is very difficult to accurately estimate these economic gains as it requires assumptions on for example the marginal economic value of a healthy person over a paralyzed polio patient or until what year you calculate these gains for. Tebbens et al. (2010)45 have attempted such a modeling exercise and arrive at a net benefit of adding GPEI vis-à-vis just national routine immunization of US-$40-50 billion in their chosen time horizon of 1988 to 2035. Such a cost benefit analysis is made even more difficult by having to extrapolate the actual case counts from reported incidence figures, which is explained in more detail in our data quality section below.
The GPEI’s estimation of eradication vs. control costs due to polio46
With 72% of polio infections not leading to any symptoms – and 99.5% of cases resulting in only temporary symptoms – it would be extremely difficult to record all cases of polio. The number of detected polio cases is lower than the total number of cases and the gap is larger when monitoring was less complete in the past.
The set-up of the Global Polio Eradication Initiative brought about a global surveillance and monitoring framework that includes the counting of only those cases that resulted in permanent paralysis. Therefore, it should be taken into account that data on polio – especially historical data – is associated with considerable mismeasurement. This greatly inhibits our ability to compare the disease burden of polio historically across countries as well.47
As we have explained above, only 0.5% of polio infections lead to permanent paralysis. It is this subgroup of polio victims with the most severe symptoms – paralysis – that is cited in most discussions regarding the prevalence of polio. But unfortunately authors do not always make this clear, and instead of referring to the ‘number of reported cases of polio’ – as it is commonly done – it would be accurate and more complete to speak of the ‘number of reported paralytic cases of polio’ when this is what is actually referred to.
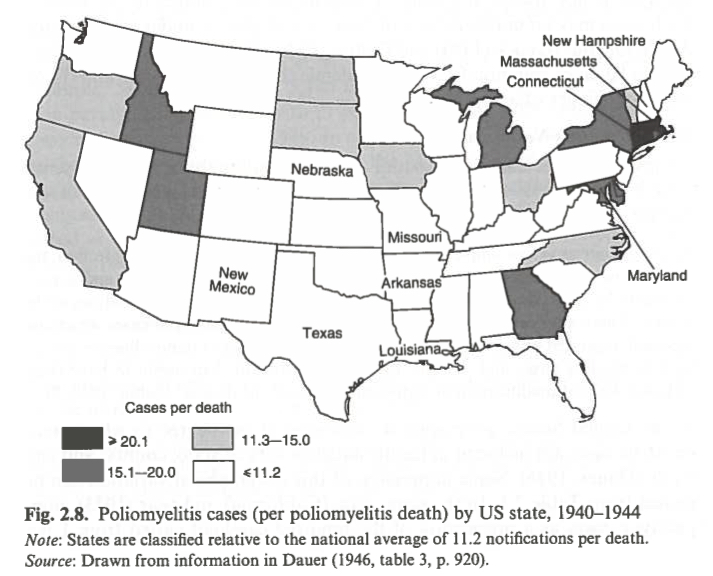
The great variance in reporting standards even within countries was already nicely illustrated by Dauer in 1946.48
He compared the number of reported deaths due to polio with the number of reported cases of polio. The reproduced map shows this ratio for each US state between 1940 and 1944. The national average of 11.2 cases per reported death was used as a benchmark and while the author acknowledges that some variation might be due to differences in virulence among different strains of the poliovirus, this variation is not powerful enough to explain such stark differences in the ratio across different parts of the USA. Instead, Dauer attributes these differences to varying reporting practices. Furthermore, in years of larger-scale polio outbreaks more non-paralytic cases are recorded than in years with smaller paralytic polio case counts.
In the past children’s paralysis was often not correctly diagnosed as polio. Stool samples need to be analyzed to be able to distinguish paralytic symptoms from Guillain-Barré Syndrome, transverse myelitis, or traumatic neuritis.49
Reported number of polio cases per polio deaths in the United States, 1940-194450
With the establishment of the Global Polio Eradication Initiative (GPEI) in 1988, monitoring and reporting procedures became standardized around the world. The GPEI’s surveillance system relies on the detection of acute flaccid paralysis (AFP), which refers to paralytic symptoms more generally and is occurring in a population for reasons other than polio, such as trauma or Guillain-Barré syndrome (GBS). Having to report all cases of AFP in any child under 15 years of age means that a country with a good monitoring system in place should detect at least 1 AFP case per 100,000 under-15-year-olds. If the number of AFP cases is less than 1 per 100,000 under-15-year-olds then there is reason to suspect that paralytic polio cases too are underreported.
Each case of AFP should be followed by a diagnosis to find its cause. Within 14 days of the onset of AFP two stool samples should be collected 24 to 48 hours apart and need to be sent to a GPEI accredited laboratory to be tested for the poliovirus.51 Since 2000, the WHO reports these surveillance quality indicators on a yearly basis for each country as the “Non polio AFP Rate” and “% Adequate Stool Collection”, respectively.
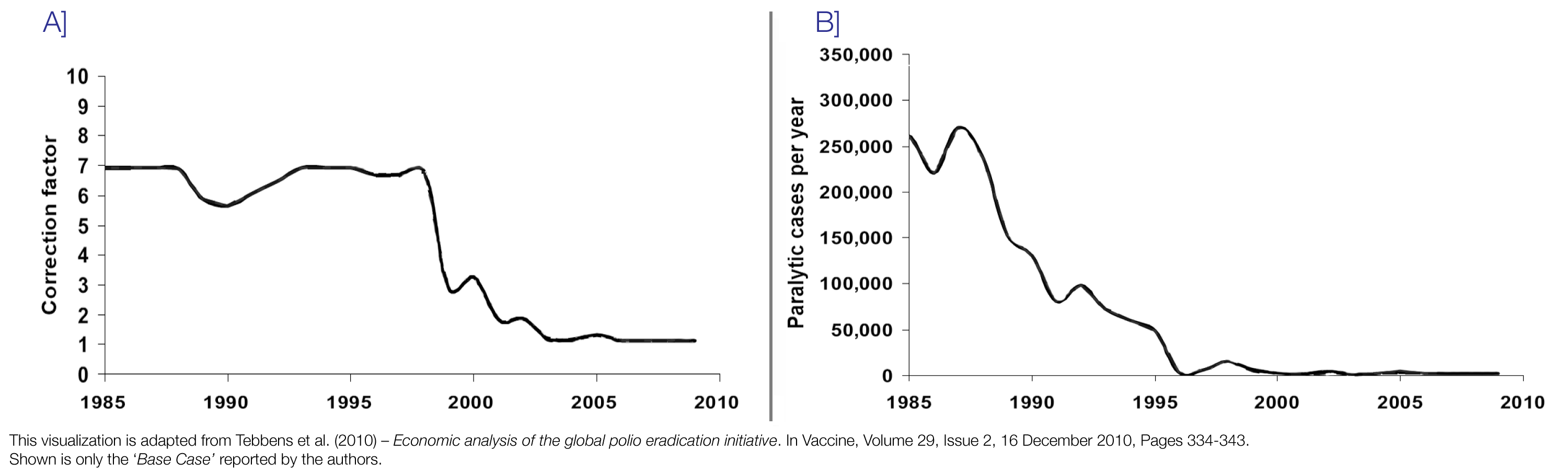
These WHO data were used for the Tebbens et al. (2011) model on the economic benefits of polio’s eradication to generate an estimate of the actual total number of paralytic polio cases based on the reported number of paralytic polio cases and a correction factor for the share of not-reported paralytic polio cases. The section A of the graphic shows the correction factor the authors used – the number of reported paralytic cases was multiplied by this factor to arrive at their estimate of the true total number of paralytic polio cases. The authors estimate that in the 1980s and 1990s the number of reported paralytic polio cases was around 7-fold lower than the true total number. One crucial piece of information for the estimation of this correction factor is that several developing countries administered surveys specifically on paralysis in the 1980s and arrived at paralytic polio case counts much higher than the initially reported figures.
As shown in section B of the graphic, Tebbens et al. (2011) estimate the total global number of paralytic polio cases to be around 270,000 per year in the late 1980s when the reported number of cases only surmounts to around 30,000 paralytic cases per year.
Many official sources, such as the Annual Report of the GPEI in 2016, cite the number of paralytic cases to be more than 350,000 in some years in the 1980s and accordingly estimate the correction factor to be even larger than 7. It is therefore even more remarkable that as reporting standards improved over time – and a greater number of paralytic polio cases are detected and reported – the number of paralytic polio cases dropped dramatically, so that only 42 paralytic polio cases were reported in 2016.
Tebben et al.’s (2011) correction factor and the resulting estimated total number of paralytic polio cases 52
- Data: Reported cases of paralytic polio (induced by the wild and the vaccine type)
- Geographical coverage: Global (by country)
- Time span: 1980-2016
- Available at: Link under Section 3.2 Polio Case Count Data of http://www.who.int/immunization/monitoring_surveillance/data/en/
- Data: Year of the last endemic case of paralytic poliomyelitis
- Geographical coverage: Global (by country)
- Time span: The earliest last case occurred in 1910, three countries still record polio cases today.
- Available at: http://polioeradication.org/where-we-work/polio-free-countries/
- Data: Immunization coverage estimates of Polio (Pol3)
- Geographical coverage: Global (by country)
- Time span: 1980-2016
- Available at: http://apps.who.int/gho/data/node.main.A831?lang=en
- Data: Polio case and death counts
- Geographical coverage: United States
- Time span: 1896-1970
- Available at: https://www.jstor.org/journal/publhealrepo1896
- Data: Polio case and death counts
- Geographical coverage: United States
- Time span: We used it from 1950 onwards
- Available at: https://stacks.cdc.gov/welcome and then select MMWR Series and limit the search to Annual publications
- Description: As the Assistant Director-General of the World Health Organization’s Polio and Emergencies Cluster, this TED talk provides a good overview over the disease, the vaccine development and the global eradication efforts of the GPEI. Though not the most recent, many of the challenges in achieving the interruption of polio transmission remain the same today.
- Date of publication: March 2011
- Available at: https://www.ted.com/talks/bruce_aylward_how_we_ll_stop_polio#t-390541
- Description: These videos give a detailed description of the virus, the disease’s epidemiology and vaccines developed against it.
- Date of publication: June 2015
- Available at: https://www.khanacademy.org/science/health-and-medicine/infectious-diseases/polio/a/what-is-polio
- Description: Roth’s story is set in 1944 Newark when a Jewish community is struck by a polio epidemic. The novel won the Man Booker Prize International in 2011 and was shortlisted for the 2011 Wellcome Trust Book Prize which rewards medical topics in literature.
- Date of publication: 5 October 2010
- Available at: Google Books in parts
- Description: This hour-long documentary illustrates the development of the polio vaccine by Dr. Jonas Salk.
- Date of publication: 2010
- Available at: Description and trailer available at the documentary producer’s page.
- Description: The 2006 winner of the Pulitzer Prize for History, this book outlines in great detail the large polio outbreaks and the successful vaccine research in the United States of the 1940s and 50s.
- Date of publication: 2005
- Available at: Partly available on Google Books.